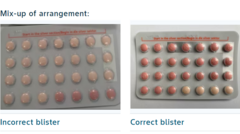
In a shocking turn of events, Bayer Ltd has initiated a recall of a batch of the widely-used Yaz Plus contraceptive pill in South Africa, following a critical packaging error. Reports indicate that some blister packs contained the wrong quantity of active and inactive pills, potentially rendering the contraception ineffective.
The South Africa Health Products Regulatory Agency confirmed the recall after it was discovered that certain packs from batch WEW96J, which is set to expire in March 2026, were incorrectly packaged. These affected packs delivered 24 hormone-free inactive pills instead of the necessary 24 hormone-containing active pills. To mitigate risks of unintended pregnancies, Bayer has urged women who have these pills to cease their use immediately and consult healthcare professionals.
To assist users, Bayer has stressed that the mix-up impacts only a confined number of packets from the specified batch. The company stated, "While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your healthcare practitioner, as they may potentially not provide the contraceptive protection you expect."
Health experts are advising anyone who has purchased the affected Yaz Plus contraceptive pills to return them to pharmacies for a replacement or full refund. Healthcare professionals, including hospitals and pharmacies, are also being instructed to return any remaining stock from this batch.
In a reassuring update, Bayer confirmed that it has identified the root cause of the mix-up and implemented corrective measures to ensure the integrity of their product moving forward. The company has also set up a dedicated helpline for further inquiries. As concerns about women's health and proper medication practices continue to grow, this incident underscores the importance of vigilant quality control in pharmaceutical manufacturing.