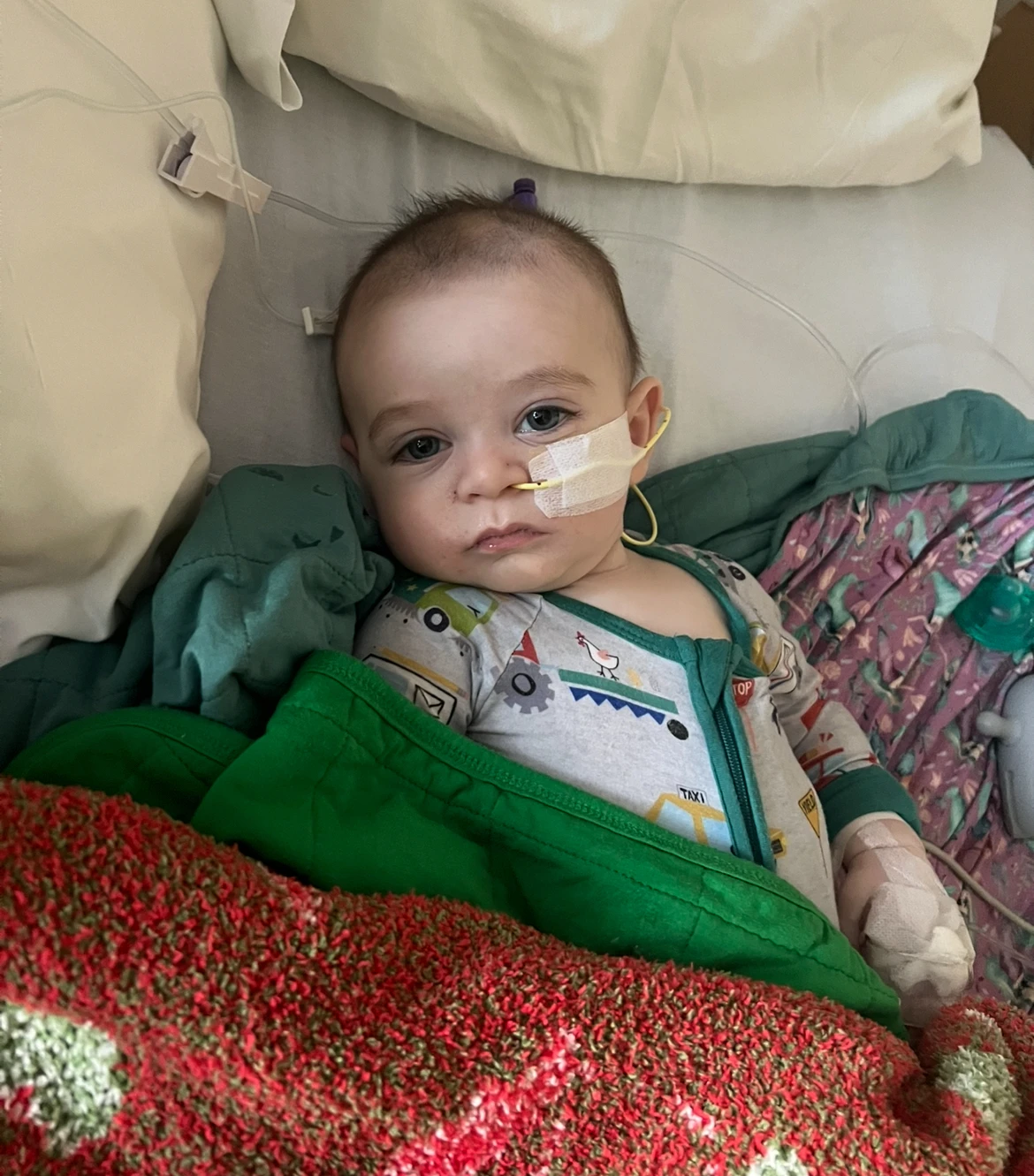
As health officials investigate over 30 cases of infant botulism linked to ByHeart baby formula since August, parents whose children suffered from similar illnesses months earlier are demanding urgent answers.
California public health officials confirmed six babies who consumed ByHeart formula were treated for botulism between November 2024 and June 2025, several months before the current outbreak which has affected at least 31 infants across 15 states.
At that time, officials claimed there was insufficient evidence to suspect a common source, and currently, they state no pre-August cases are connected to the outbreak. However, parents of at least five children who were treated for the rare and potentially fatal illness express frustration.
One mother, Amy Mazziotti, recounted how her 5-month-old son fell ill and was treated for botulism after consuming ByHeart formula earlier this year.
Another mother, Katie Connolly, revealed her daughter was hospitalized for the same reason in April. Both mothers are seeking answers as they feel the earlier cases might not have received adequate attention.
The history of botulism cases in infants typically associates with environmental spores or contaminated honey, but the connection to ByHeart formula is now under serious scrutiny following a nationwide recall on November 11 due to the rising infection cases.
Lab tests on unopened ByHeart formula have detected contamination with the bacteria that causes infant botulism, further alarming parents like Mazziotti who initially viewed the situation as a coincidence.
Legal representatives for affected families have highlighted additional cases of infants treated for botulism after consuming ByHeart formula, bolstering claims that earlier cases should be part of the investigation.
The CDC acknowledges earlier botulism reports but is focusing on the spike in cases since August. Officials suggest that the absence of lot numbers or product cans kept by parents may complicate linking these earlier cases back to the manufacturer.
Connolly feels a diverse investigation is necessary, questioning why cases from earlier months did not ignite the same concern as the recent surge. Health officials note the sudden increase in cases prompts a reevaluation of the product’s safety.
The situation leaves many parents feeling neglected as they await answers about how their infants became ill from a product they trusted. The appeal for clarity and support reflects wider concerns regarding infant safety and manufacturing accountability.