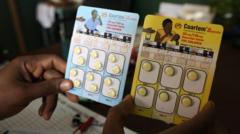
The fight against malaria, a leading cause of death among children under five, has taken a hopeful turn with the approval of the first dedicated treatment specifically designed for infants. The new medication, developed by Novartis and known as Coartem Baby (or Riamet Baby in some regions), is anticipated to be deployed in African nations within weeks, targeting the youngest and most vulnerable patients.
Historically, infants diagnosed with malaria were treated with drugs formulated for older children, which posed significant risks due to potential overdose. As babies under 4.5 kg (approximately 10 lbs) have developing liver function and process medicines differently, the absence of tailored treatments created a critical "treatment gap." In 2023 alone, malaria was associated with around 597,000 deaths, with the highest toll occurring among young children.
Novartis's Chief Executive, Vas Narasimhan, emphasized the importance of this milestone, stating that for over three decades, the company has remained committed to advancing scientific breakthroughs to combat malaria. The new drug was developed in collaboration with the Medicines for Malaria Venture (MMV), a not-for-profit organization backed by international governments and foundations. Eight African nations participated in the drug's clinical assessment, allowing them access to this necessary treatment promptly.
Martin Fitchet, CEO of MMV, heralded the development as a vital step toward addressing the devastating impact of malaria, especially among children. Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, stressed that this innovation could significantly reduce the death rate from malaria for the most affected demographics, particularly in sub-Saharan Africa. Furthermore, Novartis's decision to distribute the drug on a not-for-profit basis aims to alleviate healthcare inequalities, ensuring that the most vulnerable populations can access the care they urgently need.