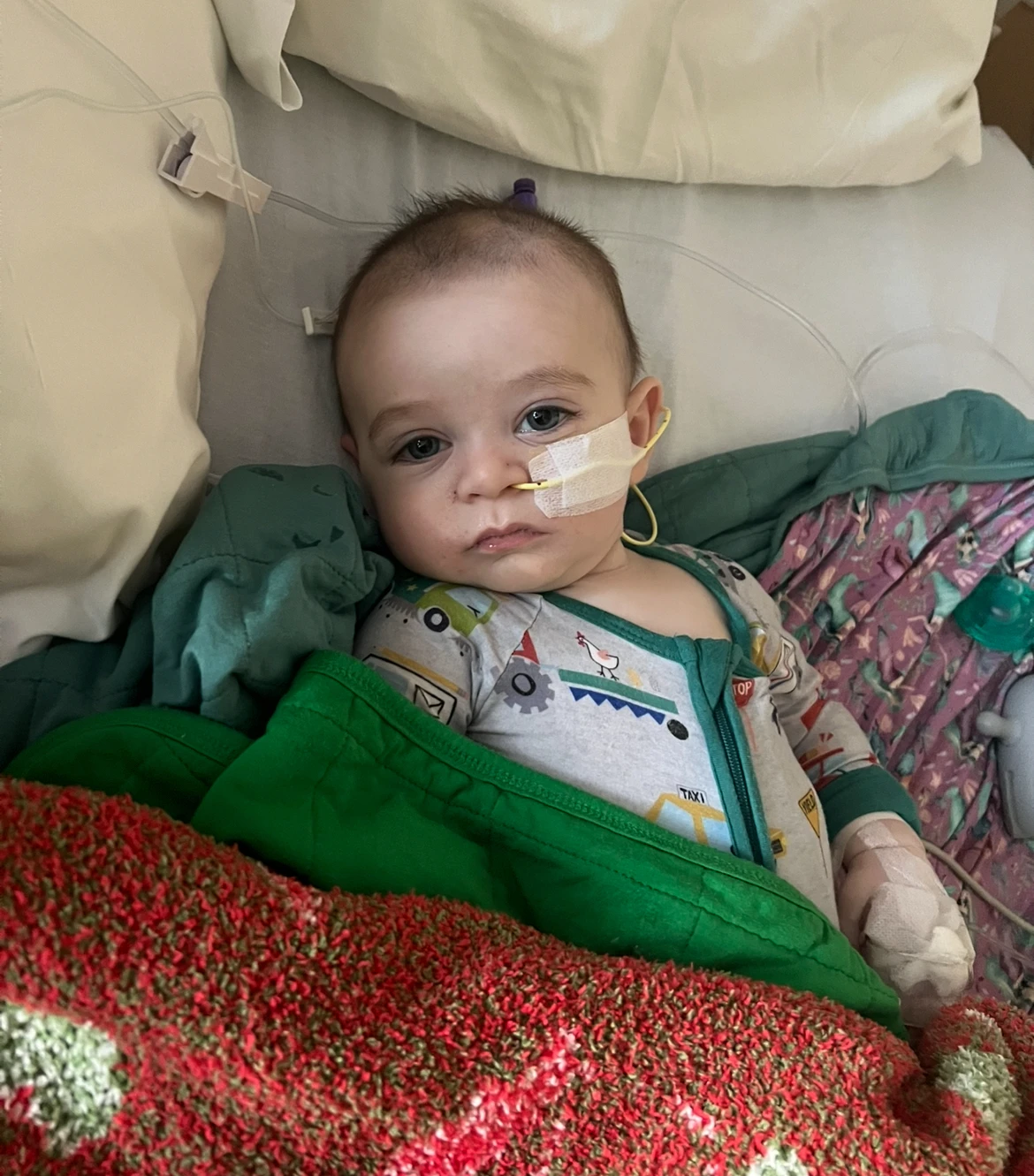
In a troubling development for parents across the United States, ByHeart infant formula has been linked to a botulism outbreak that has left dozens of infants ill. Recent laboratory tests on 36 samples from three different lots of ByHeart formula revealed contamination with the Clostridium botulinum bacteria.
According to a statement from the company, Based on these results, we cannot rule out the risk that all ByHeart formula across all product lots may have been contaminated. At least 31 infants from 15 states have reported botulism symptoms after consuming the formula since August, raising serious concerns among health officials.
This recent outbreak echoes additional incidents where infants treated for botulism were identified as having consumed ByHeart formula as early as November 2024, although those cases were not included in the current outbreak statistics. The bacteria involved can cause severe illness, particularly in infants under one year old, making this situation particularly alarming for new parents.
Botulism occurs when infants ingest spores that germinate in their intestines and produce a toxic substance. Symptoms can include sustained constipation, a weakened ability to suck or feed, drooping eyelids, and muscle weakness. Immediate medical intervention is critical as delays can lead to severe health risks.
As part of the response, ByHeart issued a nationwide recall on November 11, instructing parents to stop feeding the affected formula to their babies. Parents are advised to monitor for symptoms that can develop up to 30 days after ingestion.
Fortunately, there are emergency treatments available, such as an intravenous medication known as BabyBIG, which has been administered to over 100 infants since the outbreak began. With less than 200 infants treated in a typical year for botulism, these numbers highlight the unusual and urgent nature of the current situation.
Parents who purchased ByHeart formula from the company’s website can request a full refund for products bought after August 1. For anyone experiencing illness believed to be linked to the contaminated formula, health officials recommend contacting an FDA consumer complaint coordinator or completing an online MedWatch form.