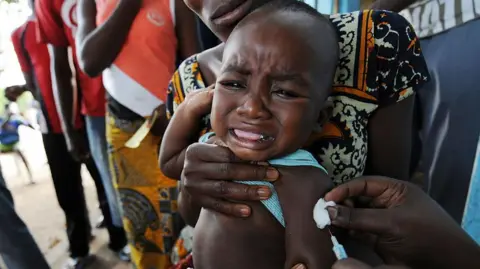
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as unethical.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age.
The WHO said it had significant concerns about the plan, and described the birth-dose vaccine as an effective and essential public health intervention, with a proven record.
The US health department, headed by Robert F Kennedy Jr, who has questioned the effects of vaccines, had sought to use the trial to answer questions about the jab's broader health effects.
The WHO on Friday raised concerns regarding the study's scientific justification, ethical safeguards and consistency with established standards for research involving humans.
It stressed that the jab had been used for more than three decades in more than 115 countries.
The WHO stated that responsible administration of the vaccine at birth would prevent newborns from suffering potentially irreversible harm.
In Guinea-Bissau, it is estimated that over 12% of the adult population has chronic Hepatitis B, with the organization noting that timely vaccination could prevent transmission from mother to child in 70-95% of cases.
Currently, infants in Guinea-Bissau receive the vaccine at six weeks, but authorities plan to introduce the birth dose nationwide by 2028.
The trial, involving a total of 14,000 babies, faced significant public outcry prompting the Guinea-Bissau government to suspend it last month.
It's not acceptable and it should not go on, said former health minister Magda Robalo, echoing widespread concern among the public about the morality of the trial. Guinea-Bissauans are not guinea pigs.